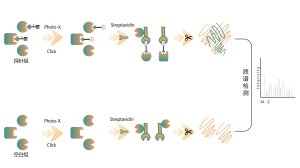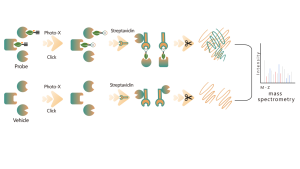Products
Cysteine Post-Translational Modifications Omics Analysis
Cysteine, with its remarkable reactivity, plays a pivotal role in protein structure and function. Serving as a nucleophilic reagent, redox catalytic center, metal ion ligand, and key site for conformational changes, it extensively participates in and profoundly influences protein activity and regulatory mechanisms. It's worth noting that cysteine residues are prone to undergo various types of post-translational modifications (PTMs), which not only finely tune protein functional properties but may also lead to functional impairment. Given the close association of such modifications with numerous major human diseases, qualitative and quantitative analysis of cysteine PTMs in proteins is of paramount importance. This holds indispensable value for in-depth understanding of the biological functions of relevant proteins and their mechanisms of action in health and disease states.
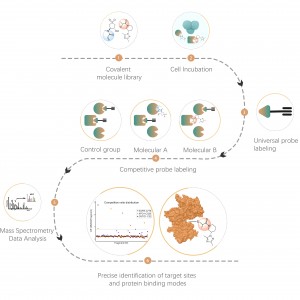
Chomix has rich experience in the identification of cysteine modifications, such as cysteine persulfidation. Innovatively utilizing universal cysteine probes based on the difference in pKa between -SH and -SSH, we adjust pH to reduce background interference from -SH, allowing the probes to predominantly label -SSH and thus effectively identify sulfenylation sites.
Chomix possesses advanced mass spectrometry technology capable of directly and accurately resolving various types of protein post-translational modifications and their specific sites. Through clever integration of separation and enrichment techniques with isotope labeling and other advanced methods, we enable large-scale, high-throughput qualitative and quantitative analysis of various modifications, providing robust technical support for in-depth research on protein post-translational modifications.

Our Advantages
1. Professional Expertise: With extensive experience and publications in leading journals, we offer tailored services for optimal results.
2. Rigorous Quality Management: Our mature quality systems adhere to ISO9001 standards, ensuring reliable reports.
3. Comprehensive Service: From probe design to bioinformatics analysis, we provide all-in-one consultation to delivery, with timely progress updates.
4. Advanced Equipment: Equipped with cutting-edge mass spectrometers like Thermo Fisher Orbitrap Exploris 480 and Bruker timsTOF, we support breakthrough research.
Our Service
| Project | Cysteine Post-Translational Modifications Omics Analysis |
| Sample | Pure protein, cell lysate, live cells, diseased tissue, blood, bacteria, plant tissue |
| Hardware Platform | Non-contact ultrasonic cell pulverizer,ChemiDoc MP Imaging System,Orbitrap Fusion Lumos Tribrid/Orbitrap Exploris 480/Q Exactive HF-X/timsTOF Pro 2 mass spectrometer |
| Project Duration | 4-8 weeks |
| Deliverables | Project Report (including experimental procedures, data analysis charts, bioinformatics analysis results) |
| Price | Click to consult |
Case Study
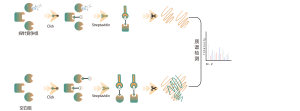
Thiolation refers to the coupling of a thiol group (-SSH) to cysteine residues in proteins, mediated by hydrogen sulfide (H2S). To investigate this process further, whole proteomes can be extracted from cell or tissue samples and labeled using a chemical probe method (cysteine-specific probe). Unlike thiol interference, this method enables effective labeling of thiol groups by precisely adjusting the pH. Subsequently, with the aid of high-resolution mass spectrometry, researchers can accurately identify sites of thiolation, thereby further elucidating the important role of this post-translational modification in vivo.
Using HeLa cell models, the whole proteome was first extracted. Subsequently, modeling with NaHS and pH adjustment allowed for accurate labeling of thiol groups by a universal cysteine probe. Through a series of steps including enrichment, enzymatic digestion, and mass spectrometric detection, 180 peptides containing -SSH modifications were identified, along with 120 related proteins, including the reported thiolation sites GAPDH_C152 and C247.

Here are the MS/MS spectra of peptides with -SSH modifications: